
This promotional hub has been developed by Bayer plc and Bayer products are discussed. Bayer has fully funded the hub and is responsible for the content, logistics and selection of speakers. OmniaMed Communications has provided editorial, marketing and logistical support. © 2023 Bayer plc
Click here for Great Britain Prescribing Information for Kerendia▼ (finerenone). Adverse Event Reporting details can be found at the bottom of the page
Downloadable resources


Menu
Downloadable resources
FIDELIO-DKD infographic
This infographic provides a visual overview of the main points from the FIDELIO-DKD study


Identification of DKD infographic
This infographic outlines the importance of urine albumin creatinine ratio (UACR) testing and monitoring of kidney function, along with pharmacotherapy options for the management of DKD
Dosing information for Kerendia
This dosing card provides details on initiating treatment with Kerendia, dose adjustment and monitoring requirements

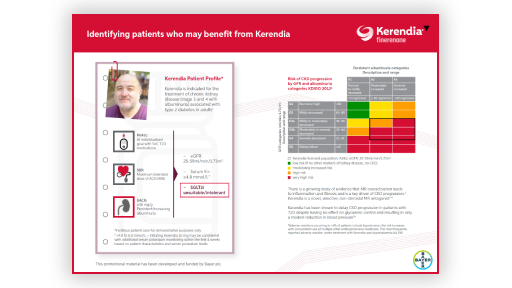
A Kerendia patient profile and Kerendia dosing
This download clearly outlines a Kerendia patient and concise dosing guidelines
Stay connected with Bayer electronic communications about Bayer’s products, services and events, some of which may be promotional in nature, by registering at pro.bayer.co.uk
Alternatively, open your phone camera application, hold it over the QR code and tap the link that appears.

Click here for Great Britain Prescribing Information for Kerendia▼ (finerenone).
Reporting adverse events and quality complaints
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Bayer plc.
If you want to report an adverse event or quality complaint, reports can be directed to:
Tel: 01182063500 or email: [email protected]
Further information is available on the “contact” tab at www.bayer.co.uk.
PP-KER-GB-0526 | September 2023



