Contraception
For women who haven’t found the right fit yet…

Contraceptive choice is a personal decision, and every woman will have her own unique needs, values and preferences.
SLYND® is a progestogen-only pill (POP) indicated for contraception – the first oestrogen-free drospirenone POP in the UK.1,2 Learn why SLYND® may be the right choice for your patients.
SLYND® offers women:
Drospirenone, which in therapeutic doses possesses mild anti-androgenic and anti-mineralcorticoid activity1,2
24/4 active/placebo dose regimen with a hormone-free interval1,3
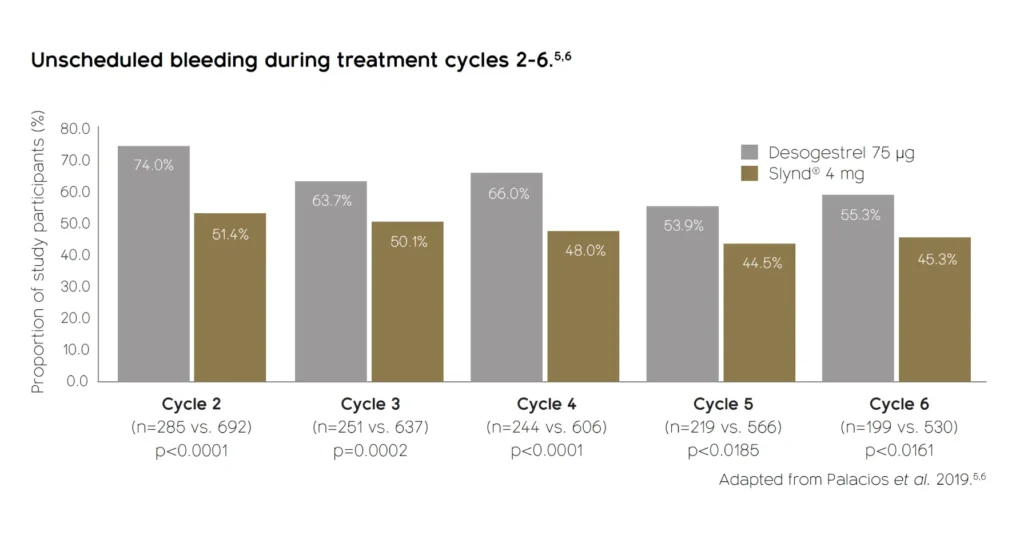
Significantly fewer unscheduled bleeding days vs. desogestrel 75 μg4-6*
The only POP in the UK with a 24-hour missed-pill window1,2
Effective contraceptive protection when used correctly1,4
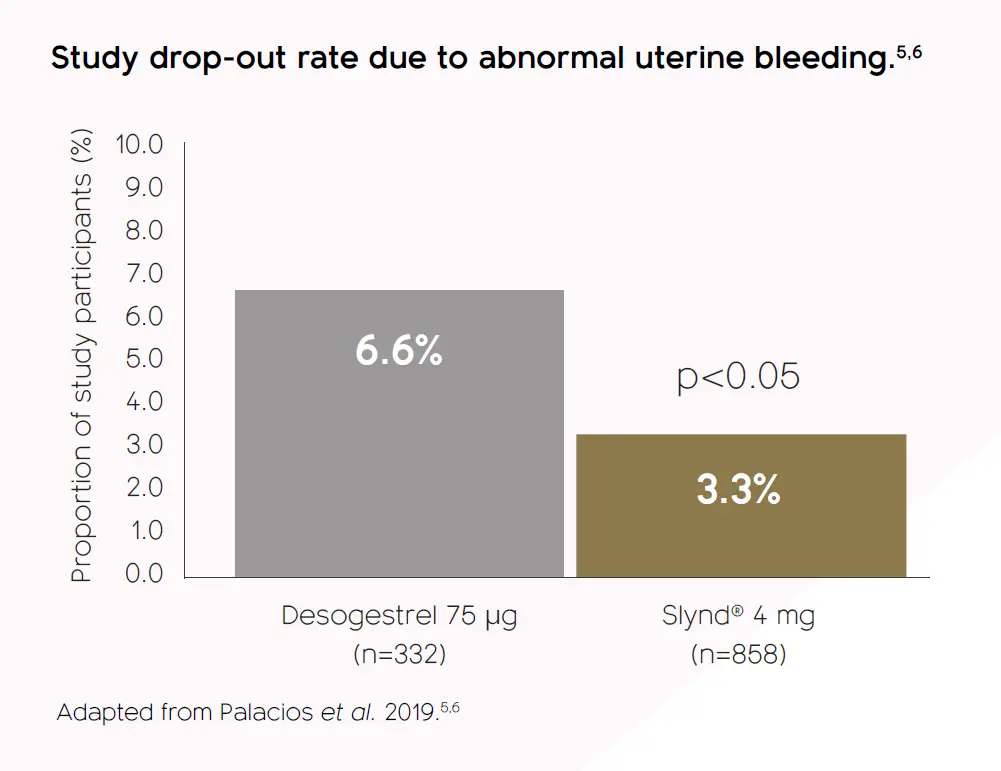
Acceptable tolerability profile, with a lower discontinuation rate vs. desogestrel 75 μg1,4-6†
SLYND® is a POP recommended as a contraceptive in the 2023 Faculty of Sexual and Reproductive Healthcare (FSRH) Guidelines and is covered by POP guidance in the UK Medical Eligibility Criteria (UKMEC).2
* Each woman’s bleeding experience with SLYND® will differ. As with all POPs, bleeding with SLYND® can be unpredictable. Women may experience scheduled bleeding (during the hormone-free interval), unscheduled bleeding, or no bleeding at all. Women report bleeding with SLYND® to be light or moderate in severity, and both scheduled and unscheduled bleeding have been seen to decrease over the first year of use.2
† SLYND® was generally well tolerated in clinical trials. Common adverse events seen with SLYND® are libido disorder, mood disturbances, headache, nausea, abdominal pain, acne, breast discomfort, metrorrhagia, vaginal haemorrhage, dysmenorrhoea, irregular menstruation, and weight increase.1
Contraception resources

45:23
ON-DEMAND
Managing bleeding issues with hormonal contraception
Dr Diana Mansour offers practical, evidence-based insights on diagnosing and managing unscheduled bleeding with hormonal contraception in primary care, including when to refer to specialist care.

Dr Diana Mansour
Consultant in Community Gynaecology, Newcastle upon Tyne, UK
Visit the Exeltis Hub for more information and educational resources on contraception
Sign up to hear from Exeltis
All fields required
AE, adverse event; POP, progestogen-only oral contraceptive pill.
References
- SLYND® (drospirenone). Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/15275/smpc#gref (Accessed September 2025).
- FSRH Clinical Guidelines progestogen-only pills. 11/2022.
- Archer DF, et al. Contraception. 2015;92(5):439–444.
- Palacios S, et al. BMC Womens Health. 2020;20:218.
- Palacios S, et al. Arch Gynae Obs. 2019;300:1805-1812.
- Palacios S, et al. PLOS ONE. 2020;15(6):e0231856.
Prescribing and adverse event reporting information:
SLYND® (drospirenone)
EXE-E/IPR-SLY-1873-v1 | September 2025