
This promotional website has been developed, organised and funded by Boehringer Ingelheim.
The media partners for this activity are GPnotebook and Diabetes on the net. GPnotebook and Diabetes on the net have had no input into the content of this website.
Prescribing information for United Kingdom and the Republic of Ireland.
Adverse event reporting information can be found at the bottom of the page.




- FOCUS-ON: RAMADAN
It is important to conduct a pre-Ramadan assessment 6–8 weeks prior to Ramadan for adults with diabetes who wish to undertake fasting during this time6
Did you know?
In a study of 12,529 Muslims with type 2 diabetes, 85.1% fasted during Ramadan despite being exempt due to their medical condition7
What should I do next?
If an adult with type 2 diabetes in your clinic still wishes to fast despite being exempt due to their medical condition, consider accessing resources from Alia Gilani that explore the steps to best support these people

Proven efficacy vs placebo1,8
Proven efficacy vs placebo:1,8 The efficacy and safety of 5 mg linagliptin was evaluated in eight phase III randomised controlled trials. Linagliptin once daily demonstrated clinically significant improvements in glycaemic control vs placebo or comparator.1
Demonstrated CV and kidney safety profile
Long-term cardiovascular and kidney safety profile of TRAJENTA® has been demonstrated in two cardiovascular outcome trials.9-15 As TRAJENTA® is primarily excreted via the bile, it is suitable for a broad range of adults with type 2 diabetes independent of renal function.1
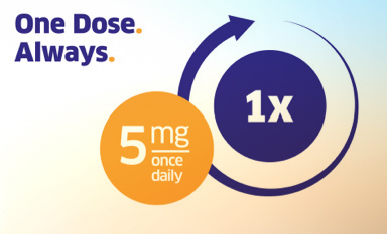
Unique convenience of one dose, once daily1
The recommended dose of TRAJENTA® for adult patients with type 2 diabetes is 5 mg once daily, independent of renal and hepatic function, body mass index, age, ethnicity, background type 2 diabetes therapy, and disease duration.1,15
- Webinar


Sarah Jarvis
Webinar Series Chair
Watch the most recent webinar
- VIRTUAL WEBINARS
Initiated and funded by Boehringer Ingelheim
PC-GB-110175 | July 2024
§ Treatment difference of 0.8% in favour of linagliptin for adjusted mean HbA1c reduction at 24 weeks between linagliptin and placebo groups.
CV: cardiovascular; SmPC: Summary of Product Characteristics.
- TRAJENTA® (linagliptin) SmPC.*
- Sitagliptin SmPC.*
- Vildagliptin SmPC.*
- Saxagliptin SmPC.*
- Alogliptin SmPC.**
- International Diabetes Federation. Diabetes and Ramadan Practical Guidelines. 2021. Available at: https://idf.org/media/uploads/2024/07/IDF_DaR_Practical_Guidelines_Ramadan.pdf (accessed November 2025).
- Hassanein M, et al. Curr Med Res Opin. 2024;40(9):1515–23.
- Del Prato S, et al. J Diab Compl. 2013;27(3):274–9.
- Rosenstock J, et al . Cardiovasc Diabetol. 2018;17(1):39.
- Rosenstock J, et al. JAMA. 2019;321(1):69–79.
- Cooper M, et al. Diabetes Obes Metab. 2020;22(7):1062–73.
- Marx N, et al. Diab Vasc Res. 2015;12(3):164–74.
- Rosenstock J, et al. JAMA. 2019;322(12):1155–66.
- Espeland MA, et al. Diabetes Obes Metab. 2021;23(2):569–80.
- Lajara R, et al. Clin Ther. 2014:36(11):1595–605.
*Summary of Product Characteristics for Trajenta, sitagliptin, vildagliptin and saxagliptin are available at: https://www.medicines.org.uk (UK) and https://www.medicines.ie/ (ROI).
**Summary of Product Characteristics for alogliptin is available at: https://www.medicines.org.uk (UK).
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as:
• monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment
• in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control
The content has been reviewed and approved by the sponsoring company prior to its publication. Editorial support for this website has been provided by
OmniaMed Communications.
PC-GB-106627 V12 | November 2025


