This promotional website has been developed, organised and funded by Boehringer Ingelheim.

Prescribing information for Great Britain and Northern Ireland and the Republic of Ireland.
Adverse event reporting information can be found at the bottom of the page.




- Focus on: Elderly and frail
DID YOU KNOW?
Compared with other age groups, the elderly and frail are at an increased risk of developing type 2 diabetes.6,7
Healthcare professionals should assess frailty in their older patients and be aware of the potential need to adjust glycaemic targets in this population.8,9

Proven efficacy vs placebo1,10
Proven efficacy vs placebo1,10: The efficacy and safety of 5 mg linagliptin was evaluated in eight phase III randomised controlled trials. Linagliptin once daily demonstrated clinically significant improvements in glycaemic control vs placebo or comparator.1
Demonstrated CV and kidney safety profile
Long-term cardiovascular and kidney safety profile of TRAJENTA® has been demonstrated in two cardiovascular outcome trials.11–17 As TRAJENTA® is primarily excreted via the bile, it is suitable for a broad range of adults with type 2 diabetes independent of renal function.1
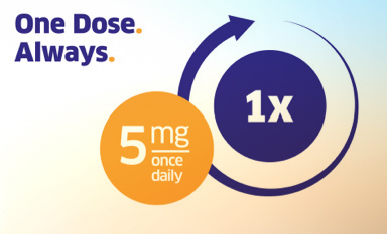
Unique convenience of one dose, once daily1
The recommended dose of TRAJENTA® for adult patients with type 2 diabetes is 5 mg once daily, independent of renal and hepatic function, body mass index, age, ethnicity, background type 2 diabetes therapy, and disease duration.1,17
- Webinar


Sarah Jarvis
Webinar Series Chair
Watch the most recent webinar
- VIRTUAL WEBINARS
Initiated and funded by Boehringer Ingelheim
PC-GB-110175 | July 2024
- TRAJENTA® (linagliptin) Summary of Product Characteristics (SmPC).*
- Sitagliptin SmPC.*
- Vildagliptin SmPC.*
- Saxagliptin SmPC.*
- Alogliptin SmPC.**
- Mendola ND, et al. NCHS data brief 2018;319:1–8.
- Kirkman MS, et al. Diabetes Care. 2012;35:2650–64.
- Strain WD, et al. Diabet Med. 2018;35:838–45.
- Strain WD, et al. Diabetes Ther. 2021;12:1227–47.
- McGill JB, et al. Diabetes Care. 2013;36:237–44.
- Rosenstock J, et al. Cardiovasc Diabetol. 2018;17:39.
- Rosenstock J, et al. JAMA. 2019;321:69–79.
- Cooper M, et al. Diabetes Obes Metab. 2020;22:1062–73.
- Marx N, et al. Diab Vasc Res. 2015;12:164–74.
- Rosenstock J, et al. JAMA. 2019;322:1155–66.
- Espeland MA, et al. Diabetes Obes Metab. 2021;23:569–80.
- Lajara R, et al. Clin Ther. 2014;36:1595–605.
*Summary of Product Characteristics for Trajenta, sitagliptin, vildagliptin and saxagliptin are available at: https://www.medicines.org.uk (GB), https://www.emcmedicines.com/en-GB/northernireland/ (NI) and https://www.medicines.ie/ (ROI).
**Summary of Product Characteristics for alogliptin are available at: https://www.medicines.org.uk (GB) and https://www.emcmedicines.com/en-GB/northernireland/ (NI).
Trajenta is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control as:
• monotherapy when metformin is inappropriate due to intolerance, or contraindicated due to renal impairment
• in combination with other medicinal products for the treatment of diabetes, including insulin, when these do not provide adequate glycaemic control
The content has been reviewed and approved by the sponsoring company prior to its publication. Editorial support for this website has been provided by
OmniaMed Communications.
PC-GB-106627 V8 | May 2024



 for treating type 2 diabetes10
for treating type 2 diabetes10