Prescribing Information and adverse events reporting information can be found at the bottom of this page.
This website has been developed and funded by A. Menarini Farmaceutica Internazionale SRL.
The media partners for this activity are GPnotebook and Diabetes on the net.

Explore the power of HbA1c control with Invokana® (canagliflozin)
Empowering control where it counts
Control of HbA1c
Significant reduction of HbA1c vs metformin, sulfonylurea, DPP4 inhibitors and other SGLT2 inhibitors, in a network meta-analysis1,2
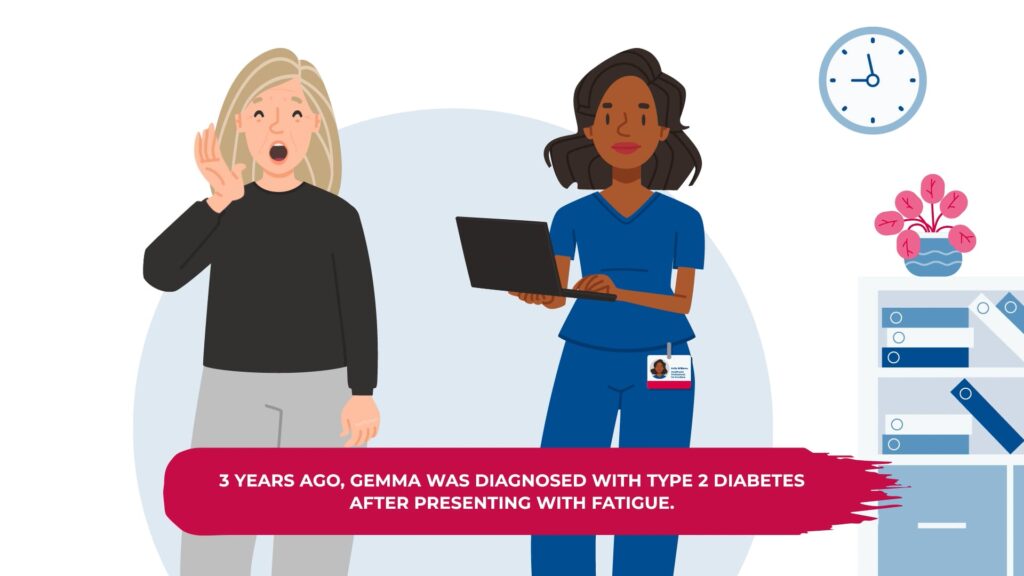
Meet Gemma

Contact your local representative to learn more about how Invokana can protect your patients living with T2D: [email protected]

Abbreviations
CV: cardiovascular; DPP-4: dipeptidyl peptidase 4; eGFR: estimated glomerular filtration rate; ESKD: end-stage kidney disease; HbA1c: glycated haemoglobin A1c; IDSC: Improving Diabetes Steering Committee; MACE: major adverse cardiovascular events; MI: myocardial infarction; T2D: type 2 diabetes.
References and footnotes
- Invokana. Summary of Product Characteristics.
- Zaccardi F, et al. Diabetes Obes Metab. 2016; 18:783–794.
- Neal B, et al. N Engl J Med. 2017;377: 644–657.
- Perkovic V, et al. N Engl J Med. 2019;380:2295–2306.
- Khanal KM, et al. PLoS ONE. 2022;17:e0271888.
- Kumar M, et al. Cureus. 2023;15:e45615.
- MACE is a composite of nonfatal stroke, nonfatal MI, and CV death.3,4
- CANVAS Program. Integrated data from two trials (CANVAS and CANVAS-R) involving a total of 10,142 participants with T2D and high CV risk. Participants in CANVAS were randomly assigned to receive canagliflozin 300 mg, 100 mg, or matching placebo; participants in CANVAS-R were randomised to canagliflozin, administered at an initial dose of 100 mg daily with an optional increase to 300 mg starting from week 13, or matching placebo. Mean follow-up was 188.2 weeks. Canagliflozin on a background of standard of care treatments for diabetes and atherosclerotic cardiovascular disease. CV (MACE: the composite of CV death, nonfatal MI, and nonfatal stroke) and renal endpoints (doubling of serum creatinine, need for renal-replacement therapy, and renal death).3
- CREDENCE Study. Randomised, double-blind, placebo-controlled, multicentre clinical trial. Patients with T2D and albuminuric chronic kidney disease received canagliflozin, at a dose of 100 mg daily or placebo. Canagliflozin on a background of standard of care treatments for diabetic kidney disease, including angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. Renal outcomes: time to first occurrence of ESKD (defined as an eGFR <15 mL/ min/1.73 m2, initiation of chronic dialysis or renal transplant), doubling of serum creatinine, and renal or CV death.4
- Based on association studies. Causality between glycaemic control and cardiovascular/renal outcomes is not directly established in the cited references.
Invokana® (canagliflozin) is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:
- As monotherapy when metformin is considered inappropriate due to intolerance or contraindications
- In addition to other medicinal products for the treatments of diabetes
For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1 of the Invokana SmPC.

PP-IN-UK-3219 | October 2025

A. Menarini Farmaceutica Internazionale SRL have had no input into the contents of the footer and are not affiliated with the links. GPnotebook is responsible for the contents of the footer.